Material Data
Detailed high-temperature thermophysical characteristics of materials are not always easy to acquire for materials, particularly those which have undergone less detailed study. Luckily for anyone who is interested in iron, very extensive research has been done on its precise characteristics under numerous conditions. Nevertheless, despite the somewhat limited nature of this data, it may prove useful.
Calculation of Enthalpy for any given temperature T:![]()
Characteristics of Iron
Symbol: Fe
Atomic number: 26
Molar weight: 55.85 g/mol
[1]
Melting point: 1809K [2]
Boiling point at 1 bar: 3158K
[2]
Heat of melting: 3670 cal/g atom (275 kJ/kg) [1]
Heat of
sublimation at 298K: 99830 cal/mol (7.48 MJ/kg) [1]
Heat of
vapourization at 298K: 83900 cal/mol (6.29 MJ/kg) [1]
Heats of formation (all at 1 bar) [2]:
Alpha at 298K: 0 kJ/mol
(reference point)
Alpha at 1184K: 33.6 kJ/mol
Gamma at 1184K:
34.5 kJ/mol
Gamma at 1665K: 51.8 kJ/mol
Delta at 1665K: 52.6
kJ/mol
Delta at 1809K: 58.6 kJ/mol
Liquid at 1809K: 72.4 kJ/mol
(1296 kJ/kg)
Gas at 298K: 413.3 kJ/mol (7400 kJ/kg)
Specific heat[2]: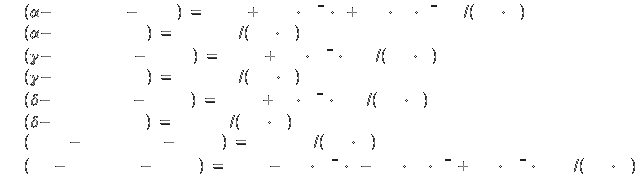
Characteristics of Nickel
Symbol: Ni
Atomic number: 28
Molar weight: 58.71 g/mol
[1]
Melting point: 1728K [1]
Boiling point at 1 bar: 3110K
[1]
Heat of melting: 4210 cal/g atom (300 kJ/kg) [1]
Heat of
sublimation at 298K: 101260 cal/mol (7.22 MJ/kg) [1]
Heat of
vapourization at 298K: 88870 cal/mol (6.34 MJ/kg) [1]
Heats of formation (all at 1 bar) [2]:
Solid at 298K: 0 kJ/mol
(reference point)
Solid at 1728K: 47.6 kJ/mol
Liquid at 298K:
17.5 kJ/mol
Liquid at 1728K: 65.0 kJ/mol
Gas at 298K: 430.1 ±
8.4 kJ/mol
Specific heat[2]: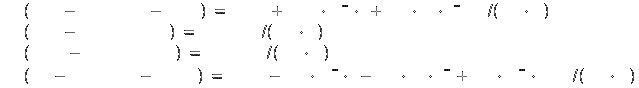
Characteristics of Silicon
Symbol: Si
Atomic number: 14
Molar weight: 28.09 g/mol
[1]
Melting point: 1685K [2]
Boiling point at 1 bar: 2950K
(estimated) [1]
Heat of melting: 11100 cal/mol (1.65 MJ/kg) [1]
Heat
of sublimation at 298K: 105000 cal/mol (15.65 MJ/kg) (estimated) [1]
Heats of formation (all at 1 bar) [2]:
Solid at 298K: 0 kJ/mol
(reference point)
Solid at 1685K: 36 kJ/mol
Liquid at 298K:
48.5 kJ/mol
Liquid at 1685K: 86.2 kJ/mol (3.07 MJ/kg)
Gas at
298K: 450 ± 8 kJ/mol (16.02 MJ/kg)
Specific heat[2]: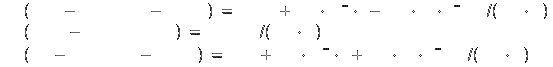
Characteristics of Carbon
Symbol: C
Atomic number: 6
Molar weight: 12.01 g/mol [1]
Melting
point: N/A (no liquid phase under normal conditions)
Boiling point
(graphite) at 1 bar: ~4000K [1]
Heat of sublimation (graphite to
monatomic gas species) at 298K: 170890 ± 500 cal/mol (59.6 MJ/kg)
(estimated) [1]
Heat of sublimation (graphite to di/triatomic gas
species) at 298K: ~200000 cal/mol (69.7 MJ/kg) (estimated) [1]
Heats of formation (all at 1 bar) [2]:
Graphite at 298K: 0
kJ/mol (reference point)
Diamond at 298K: 1.9 kJ/mol
Gas at
298K: 716.7 ± 0.5 kJ/mol
Specific heat[2]: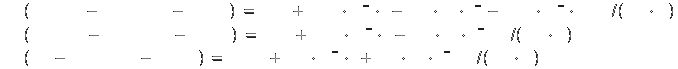
Characteristics of Water
Heats of formation (all at 1 bar) [2]:
Liquid at 298K: -285.8
kJ/mol
Gas at 298K: -241.8 kJ/mol
Specific heat[2]:![]()
Characteristics of Basalt
Major constituents (according to the US Geological Survey):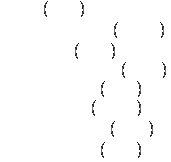
Of those constituents, the first three comprise roughly three quarters of the mass, depending on what part of the world the basalt sample is taken from.
Characteristics of Silica
Heats of formation (all at 1 bar) [2]:
Alpha-Cristobalite at
298K: -908.3 kJ/mol
Alpha-Cristobalite at 543K: -895.1
kJ/mol
Beta-Cristobalite at 543K: -893.8 kJ/mol
Beta-Cristobalite
at 1079K: -858.2 kJ/mol
Gamma-Cristobalite at 1079K: -856.2
kJ/mol
Gamma-Cristobalite at 2001K: -789.1 kJ/mol
Alpha-Quartz
at 298K: -910.9 kJ/mol
Alpha-Quartz at 847K: -876.4
kJ/mol
Beta-Quartz at 847K: -875.7 kJ/mol
Alpha-Trydimite at
298K: -910 kJ/mol
Alpha-Trydimite at 390K: -905.5
kJ/mol
Beta-Trydimite at 390K: -905.3 kJ/mol
Beta-Trydimite at
500K: -898.9 kJ/mol
Gamma-Trydimite at 500K: -898.7 kJ/mol
Liquid
at 2001K: -779.5 kJ/mol
Gas at 298K: -305.4 ± 33.5 kJ/mol
Specific heat[2]:
Characteristics of Aluminum Oxide
Heats of formation (all at 1 bar) [2]:
Alpha (Corundum) at
298K: -1675.7 ± 1.2 kJ/mol
Gamma at 298K: -1656.9 ±
6.3 kJ/mol
Delta at 298K: -1666.5 ± 4 kJ/mol
Kappa at
298K: -1662.3 ± 4 kJ/mol
Liquid at 298K: -1620.6
kJ/mol
Specific heat[2]: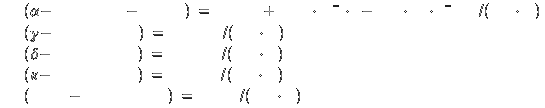
Characteristics of Iron Oxide
Heats of formation (all at 1 bar) [2]:
Solid at 298K: -266
kJ/mol
Liquid at 298K: -249.5 kJ/mol
Gas at 298K: 251 ±
20.9 kJ/mol
Specific heat[2]: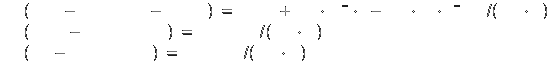
References
Thermodynamic Properties of the Elements, D.R. Stull and G.C. Sinke, American Chemical Society, 1956.
Thermochemical Data of Elements and Compounds, M. Binnewies and E. Milke, Wiley-VCH, 1999.